Our Fisheries and Mariculture Laboratory (FAML) has a long history of conducting research aimed at bringing species into culture for the first time by developing protocols for captive spawning and larval rearing. Our approach is one of basic research that makes use of our expertise in physiology, behavior, and ecology, rather than more production-oriented activities. We focus on recreationally and commercially important fish species of the Gulf of Mexico. We were the first to spawn spotted seatrout, red drum, red snapper, and southern flounder in captivity, and the first to spawn cobia that were reared from wild-caught juveniles. We developed the protocols for mass rearing of the larvae of these species, which led to commercial production of some of these species as well as stock-enhancement programs in several states in the U.S. Our broodstock also provide eggs and larvae to support our other studies of fish physiology and ecology at FAML.
Spawning & Broodstock Management
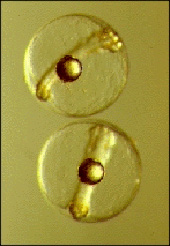
Captive spawning
We are developing protocols for year-round captive spawning of recreational and commercially important fishes, including:
- red drum (Sciaenops ocellatus)
- southern flounder (Paralichthys lethostigma)
- pigfish (Orthopristis chrysoptera)
We aim to use manipulations of environmental conditions to induce spawning and minimize the use of hormones.
Broodstock diets
We are measuring the transfer of fatty acids from the diet of broodstock to the eggs they produce. Since essential fatty acids can only be obtained through the diet, broodstock diet determines the fatty acid profile of eggs. This is important because larval quality and larval performance have been linked to fatty acid composition of eggs. We are working with Texas Parks & Wildlife Department to transfer these results to the state hatchery program to improve their stock-enhancement efforts.
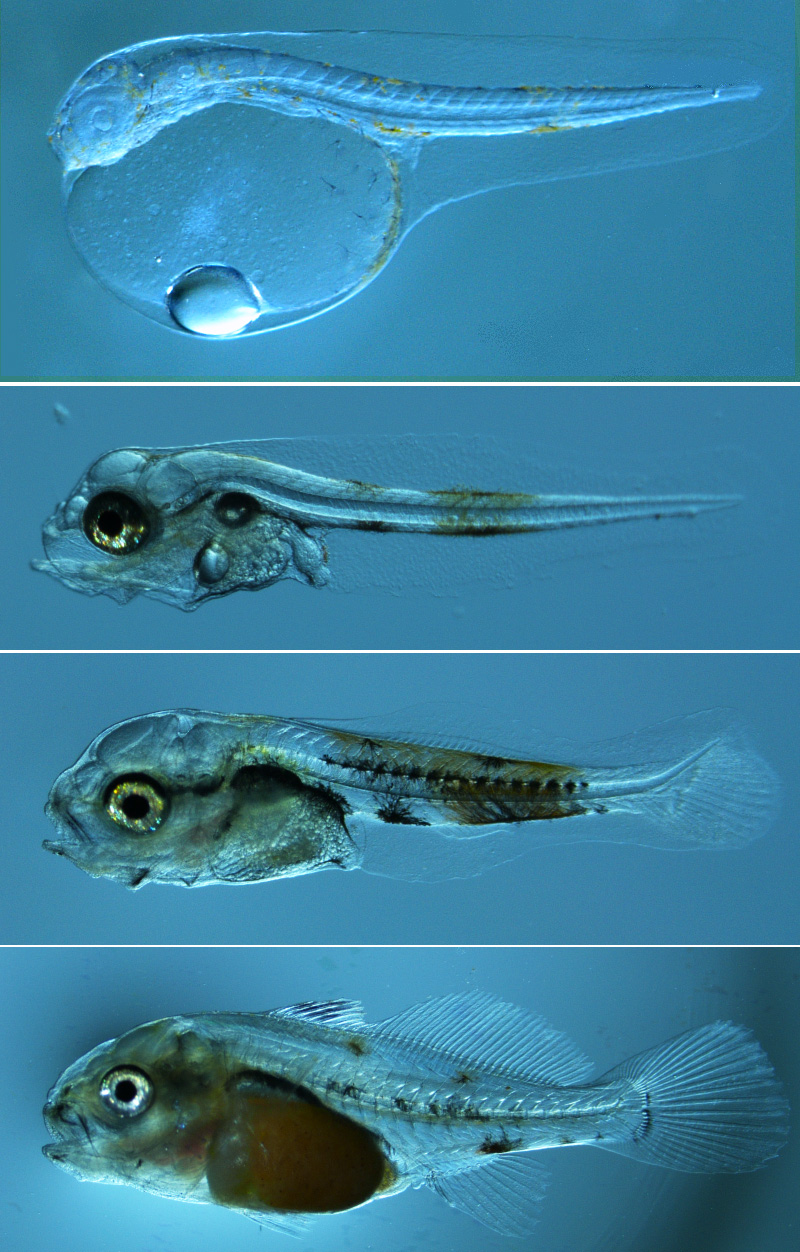
Larval & Juvenile Rearing
The aim of our work with larval and juvenile fishes is to improve rearing protocols for marine fish larvae using the eggs obtained from our captive broodstock. This research will help improve the quality and decrease cost of production of fishes reared for food production, stock-enhancement, and as baitfish.
Environmental requirements
We initially determine optimal environmental conditions for maximizing growth and survival throughout the culture period. This includes consideration of temperature, salinity, fish density, prey concentrations, green water techniques, and other conditions. Importantly, environmental requirements often change during development.
First-feeding larvae
We are working on optimizing larval feeding protocols. The species we work with do not readily accept artificial diets at first-feeding. Instead, they require live prey. Traditionally, this entails the use of rotifers and Artemia as food. These live foods, however, lack certain highly unsaturated fatty acids and other nutrients that are necessary for normal development and fast growth. We are developing protocols to enrich rotifers and Artemia with live algae, algal paste, and/or lipid emulsions. We are also investigating the use of alternative live food, such as copepod nauplii, ciliates, and dinoflagellates for use at first feeding. These natural foods may contain a more nutritionally complete diet and be a smaller, more appropriate food size for marine fish larvae at first feeding.
Larval digestive capacity
Marine fish larvae typically have rudimentary digestive tracts at first feeding. However, a well developed system is required to efficiently digest and absorb artificial feeds. We work to assess the digestive abilities of fish larvae and the changes during development by quantifying production of various digestive enzymes, including trypsin, pepsin, amylase, and lipase. This information guides new protocols for weaning larvae from live feeds to more complex and complete artificial diets.
Early weaning
Production and enrichment of live prey for rearing fish larvae is time consuming, unreliable, and expensive. We are developing protocols for early weaning of fish larvae onto artificial diets. Once we establish an early weaning protocol, we compare different diet compositions for their impact on larval growth and survival through metamorphosis to the juvenile stage.
Investigators
- Lee A. Fuiman
- Cynthia K. Faulk
More about our research:
- Diet in Fish Affects Offspring's Metabolism
- Healthy Eggs
- The Fish and the Egg: Toward a New Strategy for Fattening Up Red Drum in Texas
- Baitfish Aquaculture: Spawning and Juvenile Requirements of Pigfish
- It's a spawn! - Our breakthrough with cobia
- U.T.'s Marine Fish Research Program - Our original successes